- Home
- 1 ) Microphone
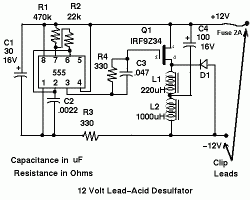
- 2 ) Battery Desulfator
- 3 ) Negative Ion Generator
- 4) TENS
- 5 ) CES
- 6 ) Solar Oven
- 7 ) Mosquito Trap
- 8 ) Home Spa
- 9 ) Weight loss tips
- 10 ) Acne Home Remedy
- 11) Dandruff Home Remedy
- 12 ) High Blood Pressure Home Remedy
- 13 ) Cold Home Remedy
- 14) Cough Home Remedy
- 15 ) Pyramidology
- 16 ) Origami
- 17 ) Website Builder / Hosting
- 18 ) Online Marketing
- 19) Forex Trading - DIY
- 20 ) E-Learning
- 21 ) Online tutoring
- 22 ) Home Gym
- 23 ) Hydroponics
- 24 ) Lecher Antenna
- 25 ) Hair styles
- 26 ) Worm Farming
- 27 ) Facials
- 28 ) Online Education
- 29 ) Biodiesel